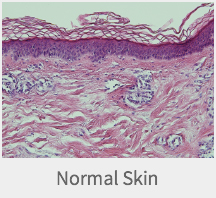
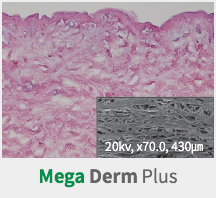
Mega Derm PlusA Cellular Dermal Matrix
- This product can carry out the functional blocks of the membrane
(soft tissue penetration protection) due to its long absorption period,
and has excellent manipulability. - Unlike other imported complete products, this product is produced
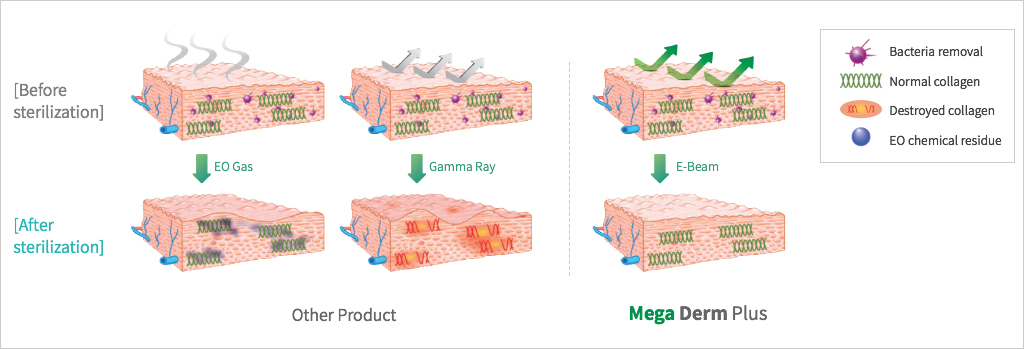
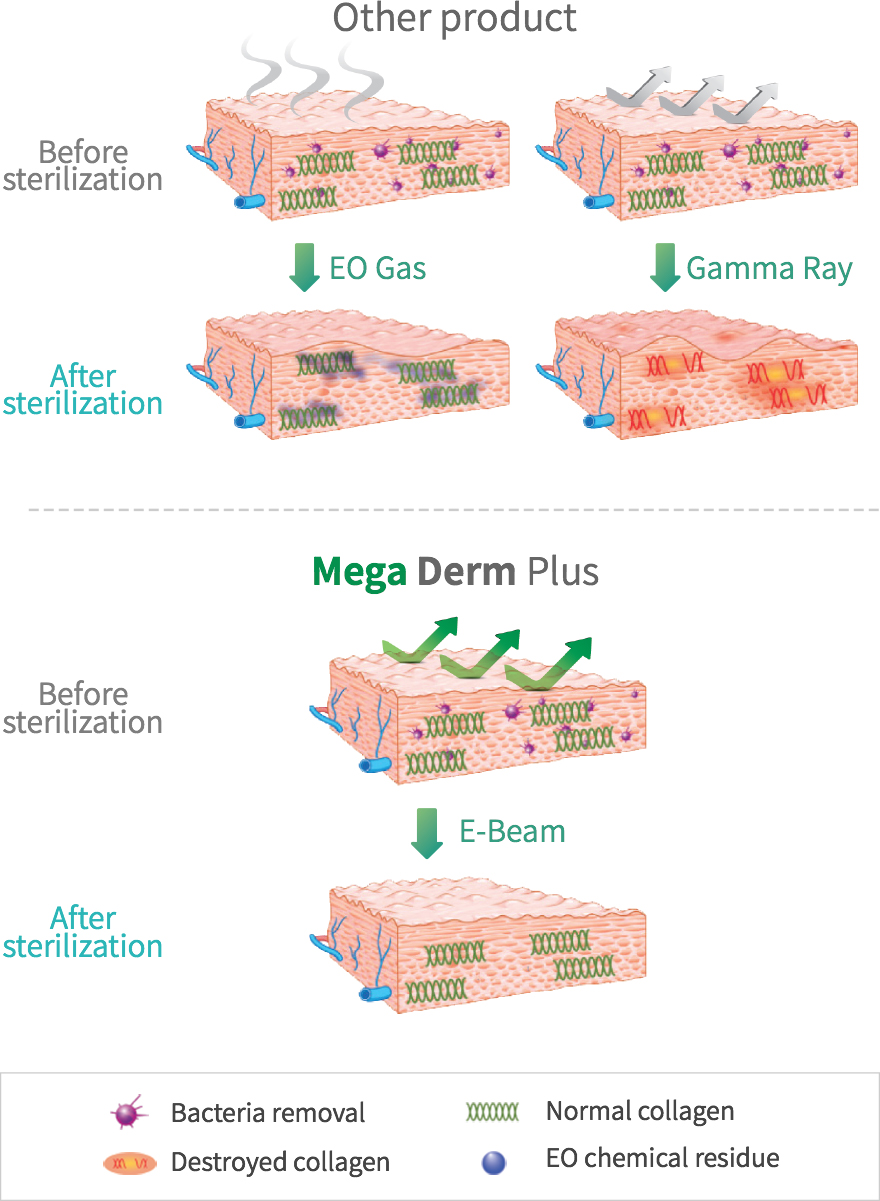
under the stringent standards of the FDA in engea. - The world's first E-Beam sterilization can induce safe and prompt
engraftment. - E-Beam is safe and can be effectively sterilized without
destroying the collagen tissue structure. - This product is the first in the world with the basement membrane layer
removed (patent pending) to maximize the transplant engraftment rate. - This shows the high engraftment rate after the transplant
by maximizing the influx of fibroblasts and/or the neovascularization.
(Patent Application No. 10-2012-0026616)
Application
- Mucogingival Defect
- Soft tissue formation around
the implant area - Wide perforation in the Schneiderian
membrane
Specifications
| Product Code | Size | Thickness |
|---|---|---|
| D1520 | 15 ~ 20mm | 0.5 ~ 0.7mm |
| D1530 | 15 ~ 20mm | 0.5 ~ 0.7mm |
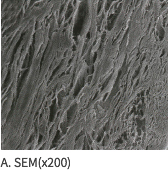
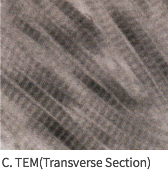
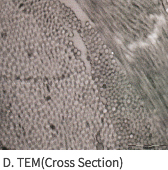
SEM Image (These have kept the collagen structure after the E-Beam sterilization.)
Mega-Derm Plus three-dimensional structure of the dermis
The world's first 'E-Beam' sterilization that does not destroy the collagen structure